
[ad_1]
About Kayla Fioravanti:
Kayla Fioravanti is the co-founder of Ology Necessities and is an award-winning creator, licensed aromatherapist and beauty formulator. She is the creator of The Artwork, Science and Enterprise of Aromatherapy and the co-author of the Amazon #1 New Launch, The Unstated Fact About Important Oils. To be taught extra about Kayla, go to her web site at: www.ologyessentials.com/
The Fact About Hand Sanitizers
In March 2020, the pandemic referred to as coronavirus (COVID-19) triggered a rush available sanitizers. Customers and companies alike quickly jumped on the DIY hand sanitizer bandwagon. Unsafe and inaccurate DIY recipes swept social media. The U.S. Meals & Drug Administration (FDA) warned customers, “FDA recommends that buyers don’t make their very own hand sanitizer. If made incorrectly, hand sanitizer will be ineffective, and there have been experiences of pores and skin burns from do-it-yourself hand sanitizer. The company lacks verifiable data on the strategies getting used to arrange hand sanitizer at house and whether or not they’re protected to be used on human pores and skin”1
Antimicrobial Sanitizers as OTC Medicine
In the USA, the Middle for Drug Analysis and Analysis (CDER), a division of the U.S. Meals and Drug Administration (FDA), regulates all antimicrobial sanitizers as over-the-counter medicine (OTC). Any and all topical merchandise making anti-microbial claims should observe all the rules. The FDA additionally regulates what sort of claims will be made on OTC hand sanitizers. The truth is, PURELL® obtained a powerful warning letter from the FDA for breaking these guidelines. On their product internet pages for PURELL® Healthcare Superior Hand Sanitizer the corporate claimed it: “Kills greater than 99.99% of most typical germs which will trigger sickness in a healthcare setting, together with MRSA & VRE.” There have been additionally extra unsubstantiated claims that transformed their OTC product into an unapproved new drug.2
In the course of the pandemic, companies additionally began making hand sanitizers with out complying with the Middle for Drug Analysis and Analysis (CDER) rules of over-the-counter (OTC) medicine. The FDA started sending out warning letters to companies who have been promoting Unapproved and Misbranded Merchandise Associated to Coronavirus Illness 2019 (COVID-19). Between March 6, 2020 and June 30, 2020 a complete of forty-three warning letters went out to firms requiring speedy cessation of all claims that misbranded merchandise in violation of the FD&C Act on social media, web site, and in all printed materials.3
The Federal Commerce Fee (FTC) additionally has regulatory energy over the claims made about merchandise. As of June 30, 2020, there have been sixty-five firms who have been cited by the FTC for breaking the FTC Act. In response to the FTC, “It’s illegal underneath the FTC Act . . . to promote {that a} product can forestall, deal with, or treatment human illness until you possess competent and dependable scientific proof, together with, when acceptable, well-controlled human scientific research, substantiating that the claims are true on the time they’re made. For COVID-19, no such research is at present identified to exist for the product recognized above. Thus, any coronavirus-related prevention or therapy claims concerning such product isn’t supported by competent and dependable scientific proof. It’s essential to instantly stop making all such claims.”5
As small enterprise homeowners it’s important that we observe the legal guidelines that govern merchandise and that we share formulation recommendation that’s fully correct and protected. The unsound formulation recommendation and promoting of unproven DIY hand sanitizers places legal responsibility on your online business for merchandise which will or could not mitigate a illness, not to mention coronavirus (COVID-19).
Momentary Steerage on Preparation and Distribution of Hand Sanitizer
In response to the hand sanitizer scarcity, the U.S. Division of Well being and Human Companies, Meals and Drug Administration, Middle for Drug Analysis and Analysis (CDER) issued a Momentary Coverage for Preparation of Sure Alcohol-Primarily based Hand Sanitizer Merchandise In the course of the Public Well being Emergency (COVID-19) Steerage for Business in March of 2020. This measure gave an avenue for companies that weren’t beforehand regulated by the FDA as drug producers to have short-term steerage on the preparation and distribution of hand sanitizer.
In response to the Momentary Coverage for Preparation of Sure Alcohol-Primarily based Hand Sanitizer Merchandise In the course of the Public Well being Emergency (COVID-19) Steerage for Business, motion is not going to be taken by the FDA during the COVID-19 public well being emergency in opposition to firms that produce hand sanitizer with very particular circumstances set by the FDA. I’ll break down the fundamentals right here, however I’ve additionally included the total textual content.
The Key Factors of the Momentary Steerage for Hand Sanitizers for Companies
The simplified key factors that anybody contemplating promoting hand sanitizer should meet embrace:
- Solely a selected listing of substances is allowed.
- The alcohol have to be denatured and meet the requirements set for within the short-term steerage.
- Solely formulation which might be in line with the World Well being Group’s (WHO) suggestions are allowed.
- Completely no energetic or inactive substances could also be used (together with important oils).
- There have to be correct file conserving of all batches.
- Testing utilizing correct strategies of research have to be run on each batch to make sure the right stage of alcohol.
- All manufacturing have to be executed underneath sanitary circumstances with acceptable tools.
- The ultimate system have to be aqueous and never be a gel, foam, or aerosol spray.
- Labeling should use the requirements for the principal show and drug panels set forth in Appendix A, B, C, or D which will be at present discovered on web page 16 of the Momentary Coverage for Preparation of Sure Alcohol-Primarily based Hand Sanitizer Merchandise In the course of the Public Well being Emergency (COVID-19) Steerage for Business
- The ability and product have to be registered within the FDA Drug Registration and Itemizing System.7
Updates to the Momentary Tips
On June 1, 2020 the FDA did an replace on the doc to additional make clear a number of particulars together with what sort of alcohols are protected to be used for hand sanitizers. This was very probably triggered by the warning letter in opposition to the corporate Eskbiochem because of the potential presence of a poisonous substance often called methanol (wooden alcohol) that’s poisonous each by pores and skin absorption and ingestion.Eight Within the up to date data, the FDA allowed for alcohol (ethanol) produced for consumption and alcohol derived from artificial processes that met United States Pharmacopeia (USP) and Meals Chemical substances Codex (FCC) grade. As well as, they made a path for alcohol produced by services that usually produce gas or technical grade alcohol (ethanol) as lengthy it’s produced by fermentation or distillated used for consumables, it accommodates no different components or chemical substances, it meets USP or FCC grade necessities, and the alcohol has been screened for impurities.
Sorts of Alcohol
The type of alcohol used is crucial to the success of a hand sanitizer system. In case you are making hand sanitizer in your private use it is very important bear in mind that rubbing alcohol, a.ok.a. isopropyl alcohol, and the alcohol that you simply drink are very totally different. The alcohol that you simply drink is ethyl alcohol (C2H5OH) and rubbing alcohol is isopropyl alcohol (C3H8O). One other alcohol that you could be see in the marketplace is denatured alcohol. It has been denatured to discourage individuals from consuming it. The Poison Management web site warns that isopropyl alcohol is toxic in small quantities to kids and likewise toxic for adults.
“Please, in case you are stockpiling alcohol for hand sanitizers, be further cautious that kids shouldn’t have entry to isopropyl alcohol.” – Kayla Fioravanti
Alcohol Content material by Numbers
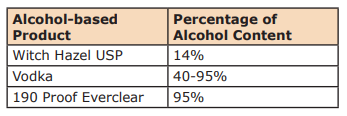
Whenever you take a look at alcohol yow will discover the proportion of alcohol in consumable liquor by checking the proof on the label. A label that reads 50% alcohol by quantity is 100-proof. Why is that this vital? One, since you should attain a minimum of the minimal stage of alcohol for the hand sanitizer to be efficient. In case your alcohol is just too weak the maths won’t ever work out. And two, you want to understand how a lot alcohol is in a completed product, but in addition the truth that water and important oils don’t combine. That is only a reality of nature. So many DIY hand sanitizers’ recipes on the web are recommending the usage of important oils. There are such a lot of issues incorrect with these recipes. One, harmful ranges of important oils are being really helpful, and two, the important oils will not be being dispersed or emulsified into the completed product. Important oils left improperly diluted and/or dispersed enhance your threat of damage or sensitization.
Don’t be discouraged by the requirements set forth within the Momentary Coverage for Preparation of Sure Alcohol-Primarily based Hand Sanitizer Merchandise In the course of the Public Well being Emergency (COVID-19) Steerage for Business. It is rather doable. My firm was in a position to register a hand sanitizer with important ease in comparison with another over-the-counter product prior to now. And when you actually wish to present hand sanitizers it’s attainable to work with a registered firm to promote it both wholesale or non-public label.
Promoting Bulk Hand Sanitizer by a Registered Firm
So, what about repackaging bulk hand sanitizer producer by a registered firm? I’ve obtained numerous questions on the legality of repackaging bulk hand sanitizers. I turned to different specialists to substantiate my suspicious. The consensus was that something repackaged can be out of compliance with the Nationwide Drug Code (NDC). The NDC is a common product identifier for human medicine in the USA. It makes use of a three-segment quantity that identifies the labeler (producer, re-packager, or distributor), the product code (figuring out energy, dosage, kind, and formulation of drug for a corporation), and the industrial package deal dimension.9 For those who select to promote the majority hand sanitizer within the packaging you buy it in, or when you’ve got the registered firm non-public label it for you, that might then meet the Nationwide Drug Code requirements.
Important Oils and COVID_19
As an aromatherapist, I merely should contact on the usage of important oils to forestall or treatment coronavirus (COVID-19). The FDA isn’t permitting the usage of any important  oils or different components within the short-term measure to any hand sanitizer. For those who beforehand have been a registered drug producer of OTC hand sanitizer and that system makes use of important oils, then you’ll be able to proceed manufacturing and promoting that product. In case you are new and utilizing the short-term measures, then you definitely can not add any materials to perfume or improve the system.
oils or different components within the short-term measure to any hand sanitizer. For those who beforehand have been a registered drug producer of OTC hand sanitizer and that system makes use of important oils, then you’ll be able to proceed manufacturing and promoting that product. In case you are new and utilizing the short-term measures, then you definitely can not add any materials to perfume or improve the system.
Additionally, remember the fact that completely no important oils have been clinically confirmed to destroy the coronavirus (COVID-19). Shannon Becker, PhD RA additional explains, “Important oils thought of to be ‘antiviral’ will not be common virus killers. Earlier than we clarify the present analysis on ‘antiviral important oils,’ it is very important make clear the distinction between virucidal and antiviral. ‘Antiviral’ implies that a compound inhibits the proliferation of a virus, whereas ‘virucidal’ means a virus is destroyed or deactivated. In lots of cases, important oils could also be efficient in killing one particular virus, however not one other. Tea tree (Melaleuca alternifolia) important oil inhibits the proliferation of influenza viruses inside cells (making it antiviral), however solely modestly inhibits HSV-1 and HSV-2 (Garozzo et al 2009). Tea tree important oil was not in a position to inhibit proliferation of the non-enveloped viruses’ poliovirus 1, adenovirus 2, echovirus 9, and Coxsackie B1 (Garozzo et al 2009).”10
Enterprise Insurance coverage for Momentary Hand Sanitizer Manufacturing and Gross sales
From one enterprise proprietor to a different, I’d be remiss to skip advising you to verify if your online business insurance coverage will enable so that you can promote this short-term OTC system. I do know that Indie Enterprise Community (IBN) members with product legal responsibility insurance coverage by IBN’s program with Veracity Insurance coverage Companies have protection for handmade hand sanitizer, as long as they’re in compliance with FDA’s Momentary Coverage for Preparation of Sure Alcohol-Primarily based Hand Sanitizer Merchandise In the course of the Public Well being Emergency. Verify along with your insurance coverage manufacturing hand sanitizer. Keep in mind that it is a short-term measure. Don’t completely revamp your online business to solely rely available sanitizer gross sales long run. It is a short-term method to pivot through the disaster brought on by the COVID 19 pandemic. Additionally, don’t make any outrageous claims. Simply hold it easy.
QUOTED DIRECTLY FROM THE FDA Momentary Coverage for Preparation of Sure Alcohol-Primarily based Hand Sanitizer Merchandise In the course of the Public Well being Emergency (COVID-19) Steerage for Business sourced from https://www.fda.gov/media/136289/obtain
1. The hand sanitizer is manufactured utilizing solely the next substances within the preparation of the product
a. Choose one in every of two choices:
(i) Alcohol (ethanol) that’s not lower than 94.9% ethanol by quantity11; OR
(ii) United States Pharmacopeia (USP grade) Isopropyl Alcohol (IPA)12,13
b. Glycerin (glycerol) USP or Meals Chemical Codex (FCC) (also referred to as “meals grade”)
c. Hydrogen peroxide.
d. Sterile water (e.g., by boiling, distillation, or different course of that leads to water that meets the specs for Purified Water USP). Water ought to be used as shortly as attainable after it’s rendered sterile or purified.
Extra Concerns for Elements in Preparation of the Product:
Alcohol (ethanol) that’s produced utilizing fermentation and distillation processes sometimes used for consumable items, and that’s made in a facility used for producing consumable items, could also be thought of to be used in hand sanitizer.
Alcohol derived from artificial processes could also be thought of to be used in hand sanitizer provided that it meets USP or FCC grade.
Alcohol produced in services usually producing gas or technical grade alcohol (ethanol) could also be thought of to be used in hand sanitizer supplied the next circumstances are current:
(i) the alcohol is produced utilizing fermentation and distillation processes sometimes used for consumable items, and no different components or different chemical substances have been added to the ethanol;
(ii) the alcohol meets USP or FCC17 grade necessities or the circumstances in
Attachment 1; and,
(iii) the alcohol has been screened for another probably dangerous impurities not
specified within the USP or FCC necessities however probably current primarily based on the
particular manufacturing setting.
Elements which might be described as solely assembly American Chemical Society (ACS) grade
requirements ought to usually not be utilized in hand sanitizers.
2. The alcohol (ethanol) is denatured both by the alcohol producer or on the level of manufacturing of the completed hand sanitizer product. Alcohol and Tobacco Tax and Commerce
Bureau rules in 27 CFR half 20 and 21, respectively, describe necessities pertaining to, and supply plenty of formulation for, denaturing alcohol. Formulation to be used in hand sanitizers underneath FDA’s short-term insurance policies embrace:
a. Components No. 40A or No. 40B with or with out the tert-butyl alcohol
b. Components No. 3C (isopropyl alcohol)
Denaturing is crucial as a result of there have been experiences of antagonistic occasions, together with deaths, from ingestion of hand sanitizer. Most experiences are from unintentional ingestion by younger kids. The alcohol ought to be denatured at both (1) the purpose of manufacturing by the alcohol manufacturing agency or (2) the purpose of manufacture or compounding of the hand sanitizer. Attachment 2 offers extra data on the formulation used to denature alcohol earlier than it’s utilized in alcohol-based hand sanitizers. Attachment 2 reproduces Appendix C from FDA steerage for business Momentary Coverage for Manufacture of Alcohol for Incorporation into Alcohol-Primarily based Hand Sanitizer Merchandise In the course of the Public Well being Emergency (COVID-19).
3. The hand sanitizer is manufactured utilizing solely the next United States Pharmacopoeia (USP) grade substances within the preparation of the product (proportion in ultimate product formulation) in line with World Well being Group (WHO) suggestions.
a. Alcohol (ethanol) (USP or Meals Chemical Codex (FCC) grade) (80%, quantity/quantity (v/v)) in an aqueous resolution denatured in accordance with Alcohol and Tobacco Tax and Commerce Bureau rules in 27 CFR half 20; or Isopropyl Alcohol (75%, v/v) in an aqueous resolution.9
b. Glycerol (1.45% v/v).10
c. Hydrogen peroxide (0.125% v/v).
d. Sterile distilled water or boiled chilly water.
4. The agency doesn’t add different energetic or inactive substances, comparable to substances to enhance the odor or style, because of the threat of unintentional ingestion in kids. Totally different or further substances could influence the standard and efficiency of the product.
5. The agency pays specific consideration to make sure the ethanol or isopropyl alcohol energetic ingredient is appropriate and the correct quantity of the energetic ingredient is used. A easy file ought to be used to doc key steps and controls to guarantee every batch matches the system developed for the drug product.
6. The hand sanitizer is ready underneath sanitary circumstances and tools utilized is nicely maintained and match for this function.
7. The agency makes use of essentially the most correct technique of research accessible on the website for verification of alcohol content material in samples of the completed drug product earlier than every batch is launched for distribution. Strategies can embrace gasoline chromatography (GC), alcoholmeter, hydrometer, or different chemical evaluation of a minimum of equal accuracy. The pattern examined will be carried out on in-process materials earlier than filling into the ultimate containers to be distributed.
8. The hand sanitizer product is produced as an aqueous resolution and never as a gel, foam, or aerosol spray. The agency packages the completed hand sanitizer product in packaging acceptable for liquid drug merchandise that may seal sufficiently to forestall evaporation of the alcohol or IPA. Handbook pump sprays that seal sufficiently to forestall evaporation are in line with this coverage.
9. The hand sanitizer is labeled in line with the connected labeling in Appendix A
(Labeling for Ethanol Formulation Shopper Use), Appendix B (Labeling for Isopropyl
Alcohol Formulation Shopper Use), Appendix C (Labeling for Ethanol Formulation
Well being Care Personnel Hand Rub Use), or Appendix D (Labeling for Isopropyl Alcohol Formulation Well being Care Personnel Hand Rub Use).
10. Corporations register their facility and listing these merchandise within the FDA Drug Registration and listing System (DRLS, https://www.fda.gov/medicine/guidance-compliance-regulatoryinformation/drug-registration-and-listing-system-drls-and-edrls). Corporations which might be required to register their international institution with FDA should listing all identified importers in the USA of their registration in accordance with Part 510(i)(1)(A) of the FD&C Act. See additionally 21 CFR 207.25(h)(2). Upon completion of registration and itemizing, corporations obtain automated affirmation from the FDA and don’t want to attend for an extra communication from FDA earlier than the agency can start to distribute these merchandise. FDA depends on registration and itemizing data to assist handle drug shortages, monitor issues of safety which will come up with product distributed to the general public, and handle product remembers, amongst different vital FDA public security actions. Our assist desk is standing by to help with facilitating this course of and will be contacted by sending an e-mail to: edrls@fda.hhs.gov.6
References
U.S & Drug Administration. “Q&A for Customers: Hand Sanitizers and COVID-19.” Accessed July 6, 2020 from: https://www.fda.gov/medicine/information-drug-class/qa-consumers-hand-sanitizers-and-covid-19
U.S & Drug Administration. “Warning Letter, GOJO Industries Inc, MARCS-CMS 599132—JANUARY 17, 2020.” Accessed July 6, 2020 from: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/gojo-industries-inc-599132-01172020
U.S & Drug Administration. “Warning Letters.” Accessed July 6, 2020 from: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/compliance-actions-and-activities/warning-letters
Federal Commerce Fee. “FTC Coronavirus Warning Letters to Firms.” Accessed July 6, 2020 from: https://www.ftc.gov/coronavirus/enforcement/warning-letters#:~:textual content=The%20function%20of%20FTC%20warning,they%20do%20not%20instantly%20cease.
Federal Commerce Fee. “Interested by making Coronavirus claims? Learn the newest FTC warning letters first.” Accessed July 6, 2020 from: https://www.ftc.gov/news-events/blogs/business-blog/2020/04/thinking-about-making-coronavirus-claims-read-latest-ftc
U.S & Drug Administration. “Momentary Coverage for Preparation of Sure Alcohol-Primarily based Hand Sanitizer Merchandise In the course of the Public Well being Emergency (COVID-19) Steerage for Business.” Accessed July 6, 2020 from: https://www.fda.gov/media/136289/obtain#web page=16
U.S & Drug Administration. “Momentary Coverage for Preparation of Sure Alcohol-Primarily based Hand Sanitizer Merchandise In the course of the Public Well being Emergency (COVID-19) Steerage for Business.” Accessed July 6, 2020 from: https://www.fda.gov/media/136289/obtain
U.S & Drug Administration. “FDA advises customers to not use hand sanitizer merchandise manufactured by Eskbiochem” Accessed July 6, 2020 from: https://www.fda.gov/medicine/drug-safety-and-availability/fda-advises-consumers-not-use-hand-sanitizer-products-manufactured-eskbiochem
Medicine. “Nationwide Drug Codes Defined.” Accessed July 6, 2020 from: https://www.medicine.com/ndc.html
Becker, Shannon, PhD RA; (2020), Tisserand Institute. “Important Oils and Coronaviruses.” Accessed July 7, 2020 from: https://tisserandinstitute.org/essential-oils-coronavirus/
[ad_2]
Source link












